In May 2022, the Center for Drug Evaluation and Research (CDER) of the U.S. Food and Drug Administration (FDA) announced the launch of a new program for expediting the development of new treatment options for rare diseases—Accelerating Rare disease Cures (ARC) Program.1 The FDA considers a disease that affects fewer than 200,000 people in the U.S. a rare disease, and estimates that more than 30 million people in the U.S. are living with a rare disease.2 However, of the approximately 7,000 known rare diseases, less than 10 percent have an FDA-approved treatment available.3
Drug development for rare diseases can be challenging for a number of reasons, including clinical trial design challenges, clinical trial recruitment, interpretation of clinical trial data in view of limited patient populations, and endpoint selection where the natural history of the disease is not well understood. Through the ARC Program, the FDA hopes to engage with different stakeholders, including patients, caregivers, advocacy groups, academics, industry, and other partners, to address the unmet medical needs of patients living with rare diseases.
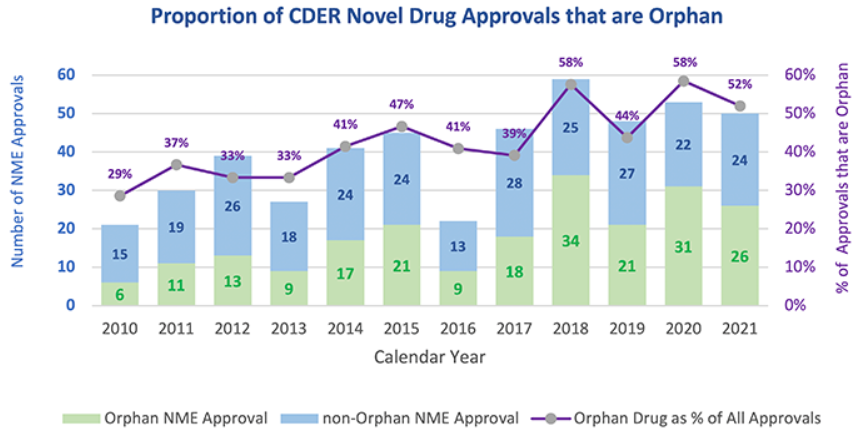
The ARC Program adds to other efforts launched by the FDA to support rare disease drug development, including the FDA's Orphan Drug (or Rare Pediatric Disease) Designation Program and the Rare Disease Cures Accelerator. Upon FDA approval, a drug product, including a biologic, with an orphan drug designation may qualify for seven years of exclusivity, or orphan exclusivity. Since 2010, there has been an increasing percentage of drugs approved by the FDA directed to a rare or "orphan" disease. In 2021, out of the 50 novel drugs approved by the FDA, more than half of those were approved for an orphan disease.
As part of the Rare Disease Cures Accelerator, in 2019, the FDA launched a data analytics platform, called the Rare Disease Cures Accelerator–Data Analytics Platform (RDCA-DAP), led by the Critical Path Institute, to establish an integrated database and analytics hub to help promote the secure sharing of existing patient-level data and standardization of new data collection.4 Researchers and drug developers can access patient-level clinical data for a particular rare disease to analyze disease progression, disease heterogeneity across the affected patient population, and other aspects of a rare disease to facilitate clinical trial design and drug development. The FDA has also developed a pilot grant program to support the development of standard core clinical outcome assessments and related endpoints for specific disease indications. Once the standard core clinical outcome assessments are developed, the FDA plans to make them publicly available at no or minimal cost.
In view of these and other FDA initiatives focused on rare diseases, we encourage drug developers focused on rare diseases to work closely with their regulatory counsel to take advantage of these programs and to engage with the FDA sooner than later to help facilitate clinical development plans and to overcome challenges that can frustrate or delay FDA approval, including challenges associated with small patient populations and clinical trial design.
For more information regarding FDA regulatory approval, orphan drug designation, rare disease initiatives at the FDA, and clinical development plans, please contact any member of Wilson Sonsini's FDA regulatory, healthcare, and consumer products practice.
Eva F. Yin contributed to the preparation of this Wilson Sonsini Alert.
[1] FDA, CDER’s ARC Program, available at https://www.fda.gov/about-fda/center-drug-evaluation-and-research-cder/cders-arc-program.
[2] FDA, CDER Launches New Accelerating Rare disease Cures (ARC) Program, available at https://www.fda.gov/drugs/drug-safety-and-availability/cder-launches-new-accelerating-rare-disease-cures-arc-program.
[3] FDA, Rare Disease Cures Accelerator, available at https://www.fda.gov/drugs/regulatory-science-research-and-education/rare-disease-cures-accelerator.
[4] Critical Path Institute, RDCA-DAP, available at https://c-path.org/programs/rdca-dap/.
Contributors
- Privacy Policy
- Terms of Use
- Accessibility